Beyond Medical Technologies Inc., through its medical face mask manufacturing subsidiary, Micron Technologies Inc., sympathizes with the worldwide wave of the Omicron Covid variant and urges its clients to utilize medical grade face masks in any social settings.
Beyond Medical Technologies Inc. (CSE: DOCT) (FSE: 7FM4) (OTC Pink: DOCKF) (“Beyond Medical” or the “Company”), through its medical face mask manufacturing subsidiary, Micron Technologies Inc. (“Micron Technologies”), sympathizes with the worldwide wave of the Omicron Covid variant and urges its clients to utilize medical grade face masks in any social settings.
Corporate Video:
https://cdn.shopify.com/s/files/1/0492/9627/7672/files/Micron-Commercial.mp4?v=1641791344
Products
Micron Technologies has been operating at its facility in Delta, British Columbia, since August 2020, where it manufactures medical grade face masks under its Medical Device Establishment License issued by Health Canada. Micron Technologies is also registered with the US Food and Drug Administration. The Company’s product line focuses on three key product lines:
Available on Amazon, Shopify, and at Walmart
Micron Technologies’ three-ply medical grade face masks and N95 Model 8800 face masks are available for purchase on Amazon, Shopify, and at Walmart. Face masks can also be purchased directly from Micron Technologies at https://micronti.com/pages/micron-video
About Beyond Medical
Beyond Medical is an industrial/technology company with a manufacturing facility located in Delta, British Columbia. The Company, through its subsidiary Micron Technologies, manufactures medical grade face masks. The Company also has an investment in digital telehealth platform in Kayan Health. Kayan Health is an artificial-intelligence powered health communications platform that allows doctors to communicate with their patients and monitor them remotely. https://kayanhealth.com
Kal Malhi, CEO
604-805-4602
kal@bullruncapital.ca
The Company is not making any express or implied claims that its products have the ability to eliminate, cure or contain COVID-19 (or SARS-2 Coronavirus) at this time.
Neither the Canadian Securities Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Canadian Securities Exchange) accepts responsibility for the adequacy or accuracy of this news release. The Canadian Securities Exchange has neither approved nor disapproved the contents of this news release.
FORWARD LOOKING STATEMENTS:
This news release includes certain statements that may be deemed “forward-looking statements”. All statements in this news release, other than statements of historical facts, that address events or developments that the Company expects to occur, are forward-looking statements. Forward-looking statements are statements that are not historical facts and are generally, but not always, identified by the words “expects”, “plans”, “anticipates”, “believes”, “intends”, “estimates”, “projects”, “potential” and similar expressions, or that events or conditions “will”, “would”, “may”, “could” or “should” occur. Although the Company believes the expectations expressed in such forward-looking statements are based on reasonable assumptions, such statements are not guarantees of future performance and actual results may differ materially from those in the forward-looking statements. Factors that could cause the actual results to differ materially from those in forward-looking statements include market prices, continued availability of capital and financing, and general economic, market or business conditions. Investors are cautioned that any such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. Forward-looking statements are based on the beliefs, estimates and opinions of the Company’s management on the date the statements are made. Except as required by applicable securities laws, the Company undertakes no obligation to update these forward-looking statements in the event that management’s beliefs, estimates or opinions, or other factors, should change.
To view the source version of this press release, please visit https://www.newsfilecorp.com/release/109477
News Provided by Newsfile via QuoteMedia
Beyond Medical Technologies (CSE:DOCT,FWB:7FM2)is a Canadian manufacturing company working to deliver essential safety equipment to protect citizens from airborne pathogens. The company’s flagship manufacturing facility is located in Delta, British Columbia, offers a fully-stable environment where it can produce technologically-enhanced personal protective equipment (PPE). Beyond Medical Technologies has invested significantly in the latest production and sterilization technology that it hopes will enable the production of high-grade protective medical equipment.
The COVID-19 crisis around the world has Beyond Medical Technologies focusing its manufacturing capacity on three core groups of users in need: hospitals, consumers and non-governmental organizations (NGOs). The company’s aim is to leverage technology to rapidly develop and produce masks and other protective equipment capable of protecting against common pathogens.
Mask use is recommended by public health authorities around the world as one of the leading means of preventing the spread of airborne viruses and diseases. During the COVID-19 pandemic, the widespread use of masks has been effective in reducing the “reproductive number” of the virus to below 1.0, a critical threshold.
“Societal norms and government policies supporting the wearing of masks by the public, as well as international travel controls, are independently associated with lower per-capita mortality from COVID-19,” concluded Virginia Commonwealth University’s Department of Ophthalmology in one study concerning mask use and global mortality rates.
Beyond Medical Technologies’ manufacturing facility in Delta, British Columbia is located close to major shipping hubs connected by rail, air and sea. The company anticipates this access to international distribution channels could allow it to quickly service regions around the world. Beyond Medical Technologies is targeting the global personal protective equipment (PPE) market, which is expected to grow to a total of US$84.7 billion by 2027 according to Grand View Research.
The company intends to continue to build out its manufacturing facility while developing proprietary products and increasing its existing portfolio through innovations and further acquisitions.
Beyond Medical Technologies prides itself on being a Canadian company with the goal of first protecting and satisfying the needs of Canadian citizens. Considering the severity of the COVID-19 pandemic, Beyond Medical Technologies is working to advance and scale its operations in order to support the global need for protective equipment as well.
The next catalyst for Beyond Medical Technologies is to secure a NIOSH N95 certification, while also exploring the potential of establishing a sterilization service for mask re-use.
Through its subsidiary Covid Technologies Inc, Beyond Medical Technologies has established its flagship 5,078-square foot manufacturing facility in Delta, British Columbia. The facility has been designed to leverage cutting-edge manufacturing technology in order to provide effective medical safety equipment to the hospitals, NGOs and consumers that need them most. The facility benefits from nearby distribution channels with the potential to quickly distribute Beyond Medical Technologies’ product by air, rail and sea.
Beyond Medical Technologies has begun acquiring mask-making equipment and materials needed for production, including an FLK120 surgical mask-making machine and over five tonnes of raw material. Through its subsidiary, Beyond Medical Technologies has secured an interim MEDL Licence (Medical Device Licence) and is working towards the production of Class 1 Protective 3ply Surgical masks, including the ASTM F2100 and F2101, neither of which require a license to manufacture.
Beyond Medical Technologies intends to begin with the development and production of surgical masks that can be used as PPE. As of Q2 2020, the company has begun the initial acquisition stage of mask filter production. Moving forward, Beyond Medical Technologies is working to acquire a melt-blown machine line capable of producing up to at least 3,200 kilograms of melt-blown polypropylene fabric per day. Beyond Medical Technologies hopes to be able to manufacture N95 Respirators and is awaiting a license from NIOSH (National Institute for Occupational Safety and Health (NIOSH).
Before becoming the CEO of Beyond Medical, Mr. Malhi founded five different companies, including Patriot One Technologies, Inc., BullRun Capital, Inc. (Canada), and Cannabix Breathalyzer, Inc.
Mr. Malhi is an entrepreneur and businessperson who previously lead 11 different companies and occupied the position of Chairman for First Responder Technologies, Inc., President, Chief Executive Officer & Director at Cairo Resources, Inc., President & Director at Algernon Pharmaceuticals, Inc., Principal at Cannabix Breathalyzer, Inc. (he founded the company), Chairman for Micron Waste Technologies, Inc. (he founded the company) and Member of Royal Canadian Mounted Police.
Zara Kanji is a founder of Zara Kanji & Associates, CPA (est. 2004). Ms. Kanji is experienced in financial reporting compliance for junior listed companies, taxation, general accounting, financial reporting and value-added advisory services for individuals, private and public companies. Ms. Kanji has served as director and officer for listed issuers providing reporting compliance services for financing and acquisitions. Additionally, Ms. Kanji is a member of the Chartered Professional Accountants of B.C. (Previously Certified General Accountants of B.C.) since August, 2003.
Beyond Medical Technologies Inc. (CSE: DOCT) (FSE: 7FM4) (“Beyond Medical” or the “Company”) is pleased to announce that it has entered into an online marketing agreement (“AGORA Agreement”) with AGORA Internet Relations Corp. (“AGORA”) and an investor marketing agreement (the “INN Agreement”) with Dig Media Inc.® dba as Investing News Network® (“INN”).
AGORA Internet Relations Corp.
Pursuant to the terms and conditions of the AGORA Agreement, AGORA will assist the Company with its launch of a 12 month online marketing campaign through AGORACOM for the purposes of targeting new potential investors that would be specifically interested in the Company’s business model, as well as engaging current shareholders. AGORA will also assist the Company with ad placements and other branding initiatives which will be posted on AGOROCOM and distributed on YouTube, various social media platforms, and all podcast platforms.
The Beyond Medical HUB, located on AGORACOM, will contain multiple landing pages, videos, photos, and other helpful information about the Company updated in real time. The Beyond Medical HUB can be located at: https://agoracom.com/ir/BeyondMedicalTechnologies. Additionally, the Company has also launched a “CEO Verified” Discussion Forum on AGORACOM to serve as the Company’s primary social media platform to interact with both current and prospective shareholders in a fully moderated environment.
The Beyond Medical discussion forum can be found at: https://agoracom.com/ir/BeyondMedicalTechnologies/forums/discussion
In connection with the services provided by AGORA under the AGORA Agreement, the Company will pay AGORA aggregate consideration of $100,000 plus applicable taxes, which shall paid via the issuance of common shares in the capital of the Company (the “Payment Shares“). The Payment Shares will be issued in five installments throughout the term of the AGORA Agreement, which commenced on November 29, 2021 and ends on December 30, 2022. The number and deemed price of the Payment Shares to be issued will be calculated using the closing price of the Company’s common shares on the Canadian Securities Exchange on each date on which the Payment Shares are to be issued.
AGORA is the pioneer of online marketing, broadcasting, conferences and investor relations services to North American small and mid-cap public companies, with more than 300 companies served. AGORACOM is the home of more than 7.7 million investors that visited 55.2 million times and read over 600 million pages of information over the last 10 years. The average visit of 8min 43sec is more than double that of global financial sites, which can be attributed to the implementation and enforcement of the strongest moderation rules in the industry. AGORA’s contact information is as follows: AGORA Internet Relations Corp., 155 East Beaver Creek Road, Unit 24, Suite 304, Richmond Hill, ON, L4B 2N1.
Investing News Network
The Company has also engaged INN, a British Columbia corporation, to provide an advertising and investor awareness campaign. INN will feature the Company’s news and logo on its channel homepage as well as use its social media channels to bring attention to the Company’s news.
The Company will pay INN aggregate consideration of $52,200 plus applicable taxes (the “INN Consideration“). The Company has agreed to pay the INN Consideration in three equal instalments of $17,400 plus applicable taxes, with the first payment payable on receipt of an invoice from INN. The second and third payments will be due on February 29, 2022 and May 29, 2022, respectively. The term of the INN Agreement is thirteen months, ending on December 30, 2022.
INN is a private company headquartered in Vancouver, Canada, dedicated to providing independent news and education to investors since 2007. INN does not provide Investor Relations or Market Making services. INN’s contact information is as follows: Dig Media Inc.® d/b/a Investing News Network®, Suite L200, 560 Beatty Street, Vancouver, BC V6B 2L3.
About Beyond Medical
Beyond Medical is an industrial/technology company with a manufacturing facility located in Delta, B.C. The Company, through its subsidiary Micron Technologies, manufactures medical grade face masks. For further information contact:
Kal Malhi, CEO
604-805-4602
kal@bullruncapital.ca
The Company is not making any express or implied claims that its products have the ability to eliminate, cure or contain COVID-19 (or SARS-2 Coronavirus) at this time.
Neither the Canadian Securities Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Canadian Securities Exchange) accepts responsibility for the adequacy or accuracy of this news release. The Canadian Securities Exchange has neither approved nor disapproved the contents of this news release.
FORWARD-LOOKING STATEMENTS:
This news release includes certain statements that may be deemed “forward-looking statements”. All statements in this news release, other than statements of historical facts, that address events or developments that the Company expects to occur, are forward-looking statements. Forward-looking statements are statements that are not historical facts and are generally, but not always, identified by the words “expects”, “plans”, “anticipates”, “believes”, “intends”, “estimates”, “projects”, “potential” and similar expressions, or that events or conditions “will”, “would”, “may”, “could” or “should” occur. Although the Company believes the expectations expressed in such forward-looking statements are based on reasonable assumptions, such statements are not guarantees of future performance and actual results may differ materially from those in the forward-looking statements. Factors that could cause the actual results to differ materially from those in forward-looking statements include market prices, continued availability of capital and financing, and general economic, market or business conditions. Investors are cautioned that any such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. Forward-looking statements are based on the beliefs, estimates and opinions of the Company’s management on the date the statements are made. Except as required by applicable securities laws, the Company undertakes no obligation to update these forward-looking statements in the event that management’s beliefs, estimates or opinions, or other factors, should change.
To view the source version of this press release, please visit https://www.newsfilecorp.com/release/106708
News Provided by Newsfile via QuoteMedia
Beyond Medical Technologies Inc. (” Beyond Medical ” or the ” Company “) (CSE: DOCT) ( Frankfurt : 7FM4) is pleased to provide the following corporate update regarding its wholly-owned subsidiary Micron Technologies Inc. (” Micron Technologies “).

“Amazon’s Choice” Designation for Medical Grade Face Masks
Micron Technologies has been operating at the Company’s facility in Delta, British Columbia since August 2020 where it manufactures three-ply medical grade face masks. In early April 2021 , Micron Technologies’ three-ply medical grade face masks achieved the “Amazon’s Choice” designation from Amazon. The “Amazon’s Choice” designation is achieved by having a high star rating, competitive price, low return rate, consistent inventory, and high sales. Micron Technologies anticipates a sales boost following achieving the “Amazon’s Choice” designation.
In addition to Amazon, Micron Technologies’ products are available online at Walmart and Shopify. Institutional customers and those who are interested in obtaining a quote for large size orders are encouraged to contact Milan@micronti.com .
Micron Technologies Accepting Bitcoin
Micron Technologies has entered the cryptocurrency space by now accepting Bitcoin as a form of payment for its products. The option to pay with Bitcoin is available to both retail and wholesale customers. Customers who wish to pay with Bitcoin can do so on Micron Technologies’ website where the payment will be completed via BitPay, a Bitcoin service provider.
About Beyond Medical
Beyond Medical is an industrial/technology company with a manufacturing facility located in Delta, British Columbia . The Company is developing its Organivore and Pharmavore waste digesters using its proprietary technology.
The Company, through its subsidiary Micron Technologies, is also manufacturing medical grade face masks.
The Company is not making any express or implied claims that its products have the ability to eliminate, cure or contain COVID-19 (or SARS-2 Coronavirus) at this time.
Neither the Canadian Securities Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Canadian Securities Exchange) accepts responsibility for the adequacy or accuracy of this news release. The Canadian Securities Exchange has neither approved nor disapproved the contents of this news release.
FORWARD LOOKING STATEMENTS:
This news release includes certain statements that may be deemed “forward-looking statements”. All statements in this news release, other than statements of historical facts, that address events or developments that the Company expects to occur, are forward-looking statements. Forward-looking statements are statements that are not historical facts and are generally, but not always, identified by the words “expects”, “plans”, “anticipates”, “believes”, “intends”, “estimates”, “projects”, “potential” and similar expressions, or that events or conditions “will”, “would”, “may”, “could” or “should” occur. Although the Company believes the expectations expressed in such forward-looking statements are based on reasonable assumptions, such statements are not guarantees of future performance and actual results may differ materially from those in the forward-looking statements. Factors that could cause the actual results to differ materially from those in forward-looking statements include market prices, continued availability of capital and financing, and general economic, market or business conditions. Investors are cautioned that any such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. Forward-looking statements are based on the beliefs, estimates and opinions of the Company’s management on the date the statements are made. Except as required by applicable securities laws, the Company undertakes no obligation to update these forward-looking statements in the event that management’s beliefs, estimates or opinions, or other factors, should change.
SOURCE Beyond Medical Technologies Inc.
![]() View original content to download multimedia: http://www.newswire.ca/en/releases/archive/April2021/27/c4406.html
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/April2021/27/c4406.html
News Provided by Canada Newswire via QuoteMedia
Beyond Medical Technologies Inc. (” Beyond Medical ” or the ” Company “) (CSE: DOCT) ( Frankfurt : 7FM4) is pleased to provide the following corporate update regarding its wholly-owned subsidiary Micron Technologies Inc. (” Micron Technologies “).

Manufacturing Three-Ply Medical Grade Face Masks at Full Capacity
Micron Technologies has been manufacturing and selling three-ply medical grade face masks at the Company’s facility in Delta, British Columbia since August 2020 . The three-ply medical grade face masks are being manufactured pursuant to Micron Technologies’ Medical Device Establishment License issued by Health Canada. The three-ply medical grade face masks offer three layers of protection, which conforms to the American Society for Testing and Materials’ F2100 Level 3 standards. The non-woven masks feature an adjustable nose clip designed to protect both front-line workers and consumers. Micron Technologies is currently operating three shifts a day to manufacture three-ply medical grade face masks.
Manufacturing N95 Face Masks
Micron Technologies is now also setting up manufacturing N95 Model 8800 face masks, which have been approved. Micron Technologies has submitted its N95 Model 8800 face masks to the National Institute for Occupational Safety and Health (” NIOSH “) and is confident it will obtain NIOSH certification as its N95 Model 8800 face masks have already passed testing with Kinetrics Analytical and Environmental Laboratories. Micron Technologies has purchased a large quantity of raw materials to manufacture N95 Model 8800 face masks and plans to maximize production efforts once NIOSH certification has been obtained. As of last month there was still a significant shortage of N95 masks and approximately ” 56 percent of frontline workers say they still don’t have enough N95 masks. ”
Third Wave of COVID-19
According to Dr. Abdu Sharkawy , an infectious disease specialist, the third wave of COVID-19 is ” likely going to be worse than the first two .” With respect to the addition of variants, Dr. Sharkawy noted ” and of course we’ve seen what these variants can offer, they can really lead to an explosion of cases very quickly .” Dr. Theresa Tam , Canada’s Chief Public Health Officer, announced a 64% increase in new cases involving variants. In addition to the new variants, the slow vaccine roll-out and complacency with public health restrictions are contributing to the third wave.
Earlier this month Canada surpassed 1,000,000 confirmed COVID-19 cases since the beginning of the pandemic, with the provinces of Ontario , Quebec , and British Columbia being hit the hardest by the third wave.
Products Now Available at Walmart
As previously announced, Micron Technologies’ three-ply medical grade face masks and N95 medical grade face masks have been approved for sale by Walmart. Micron Technologies expects its products to be available at www.walmart.com in the coming weeks.
In addition to Walmart, Micron Technologies’ products are also available on Amazon and Shopify. Institutional customers and those who are interested in obtaining a quote for large size orders are encouraged to contact Milan@micronti.com . Customers can also make orders directly at https://micronti.com .
About Beyond Medical
Beyond Medical is an industrial/technology company with a manufacturing facility located in Delta, British Columbia . The Company is developing its Organivore and Pharmavore waste digesters using its proprietary technology. The Company, through its subsidiary Micron Technologies, is also manufacturing medical grade face masks.
The Company is not making any express or implied claims that its products have the ability to eliminate, cure or contain COVID-19 (or SARS-2 Coronavirus) at this time.
Neither the Canadian Securities Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Canadian Securities Exchange) accepts responsibility for the adequacy or accuracy of this news release. The Canadian Securities Exchange has neither approved nor disapproved the contents of this news release.
FORWARD LOOKING STATEMENTS:
This news release includes certain statements that may be deemed “forward-looking statements”. All statements in this news release, other than statements of historical facts, that address events or developments that the Company expects to occur, are forward-looking statements. Forward-looking statements are statements that are not historical facts and are generally, but not always, identified by the words “expects”, “plans”, “anticipates”, “believes”, “intends”, “estimates”, “projects”, “potential” and similar expressions, or that events or conditions “will”, “would”, “may”, “could” or “should” occur. Although the Company believes the expectations expressed in such forward-looking statements are based on reasonable assumptions, such statements are not guarantees of future performance and actual results may differ materially from those in the forward-looking statements. Factors that could cause the actual results to differ materially from those in forward-looking statements include market prices, continued availability of capital and financing, and general economic, market or business conditions. Investors are cautioned that any such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. Forward-looking statements are based on the beliefs, estimates and opinions of the Company’s management on the date the statements are made. Except as required by applicable securities laws, the Company undertakes no obligation to update these forward-looking statements in the event that management’s beliefs, estimates or opinions, or other factors, should change.
SOURCE Beyond Medical Technologies Inc.
![]() View original content to download multimedia: http://www.newswire.ca/en/releases/archive/April2021/07/c7788.html
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/April2021/07/c7788.html
News Provided by Canada Newswire via QuoteMedia
Beyond Medical Technologies Inc. (” Beyond Medical ” or the ” Company “) (CSE: DOCT) ( Frankfurt : 7FM4) is pleased to provide the following corporate update regarding its wholly-owned subsidiary Micron Technologies Inc. (” Micron Technologies “).

Products Now Available at Walmart
Micron Technologies’ three-ply medical grade face masks and N95 medical grade face masks have been approved for sale by Walmart. Micron Technologies has completed the onboarding process and its products will be available at www.walmart.com by the beginning of April 2021 .
Kal Malhi , CEO of Beyond Medical states: “We are pleased to offer our products to loyal Walmart customers at competitive prices. The availability of our product with one of the largest retailers in the world will bring significant exposure to our made-in- Canada medical face mask brand. Our recently completed capital raise will allow us to ramp up manufacturing to meet new demand from Walmart customers.”
In addition to Walmart, Micron Technologies’ products are also available on Amazon and Shopify. Institutional customers and those who are interested in obtaining a quote for large size orders are encouraged to contact Milan@micronti.com .
Three-Ply Medical Grade Face Masks
Micron Technologies has been manufacturing and selling three-ply medical grade face masks since August 2020 . The masks are available in the colour blue and black. The majority of Micron Technologies’ sales have been to provincial governments; however, sales are also being completed online to various other customers. Micron Technologies is considering acquiring additional manufacturing equipment and hiring more personnel as it is currently operating at full capacity and continues to sell out of inventory.
N95 Face Masks
In addition to the three-ply medical grade face masks, Micron Technologies is also manufacturing N95 Model 8800 face masks. Micron Technologies’ N95 Model 8800 face masks have been approved by Health Canada and the US Food and Drug Administration. Additionally, the N95 Model 8800 face masks have passed testing with Kinetrics Analytical and Environmental Laboratories. Accordingly, Micron Technologies is confident that its N95 Model 8800 face masks will obtain medical-grade certification from the National Institute for Occupational Safety and Health in the coming weeks. There are currently a limited number of NIOSH approved medical face mask manufacturers and the demand for these masks in outpacing supply.
On February 2,2012 , Washington Post reported on N95 masks: “The initial shortage has eased, but there still aren’t enough medical masks for health-care workers, let alone others. Demand for N95s is 500 to 1,000 percent higher than it was a year ago, said Megan Ranney , co-founder of Get Us PPE, which helps front-line workers obtain personal protective equipment.”
Last month President Biden’s chief medical advisor for Covid-19, Dr. Anthony Fauci , indicated that Americans may still be wearing masks in 2023, as mask-wearing has been critical in slowing down the spread Covid-19. N95 masks are the gold standard of personal protective equipment, particularly among healthcare professionals as they provide the highest level of protection for the user and those in close proximity. Beyond Medical hopes to have its N95 Model 8800 face masks certified as soon as possible in an effort to reduce the spread of Covid-19.
About Beyond Medical
Beyond Medical is an industrial/technology company with a manufacturing facility located in Delta, BC . The Company is developing its Organivore and Pharmavore waste digesters using its proprietary technology. The Company, through its subsidiary Micron Technologies, is also manufacturing medical grade face masks.
The Company is not making any express or implied claims that its products have the ability to eliminate, cure or contain COVID-19 (or SARS-2 Coronavirus) at this time.
Neither the Canadian Securities Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Canadian Securities Exchange) accepts responsibility for the adequacy or accuracy of this news release. The Canadian Securities Exchange has neither approved nor disapproved the contents of this news release.
FORWARD LOOKING STATEMENTS:
This news release includes certain statements that may be deemed “forward-looking statements”. All statements in this news release, other than statements of historical facts, that address events or developments that the Company expects to occur, are forward-looking statements. Forward-looking statements are statements that are not historical facts and are generally, but not always, identified by the words “expects”, “plans”, “anticipates”, “believes”, “intends”, “estimates”, “projects”, “potential” and similar expressions, or that events or conditions “will”, “would”, “may”, “could” or “should” occur. Although the Company believes the expectations expressed in such forward-looking statements are based on reasonable assumptions, such statements are not guarantees of future performance and actual results may differ materially from those in the forward-looking statements. Factors that could cause the actual results to differ materially from those in forward-looking statements include market prices, continued availability of capital and financing, and general economic, market or business conditions. Investors are cautioned that any such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. Forward-looking statements are based on the beliefs, estimates and opinions of the Company’s management on the date the statements are made. Except as required by applicable securities laws, the Company undertakes no obligation to update these forward-looking statements in the event that management’s beliefs, estimates or opinions, or other factors, should change.
SOURCE Beyond Medical Technologies Inc.
![]() View original content to download multimedia: http://www.newswire.ca/en/releases/archive/March2021/30/c9088.html
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/March2021/30/c9088.html
News Provided by Canada Newswire via QuoteMedia
Beyond Medical Technologies Inc. (” Beyond Medical ” or the ” Company “) (CSE: DOCT) (FSE: 7FM4) thru its subsidiary Micron Technologies Inc is pleased to provide the following corporate update.

THREE PLY MEDICAL GRADE FACE MASKS:
Micron Technologies, the company’s wholly owned subsidiary has been manufacturing and selling medically certified three ply face masks in the colour blue and also in black since August 2020 . The company is selling out all the inventory that it can manufacture on three shifts a day and is looking to bring in additional manufacturing equipment and personnel. Parties wishing to purchase our masks online can do so at: www.micronti.com
N95 MASKS
There is an ongoing shortage of N95 medical grade masks for frontline first responders across North America .
Ranvir Gujral stated on NPR radio “But fewer companies shifted to making N95 masks, which are far more complicated, and they’re in short supply. Nurses and doctors are re-using masks over and over again. Some small medical practices can’t even find supplies they can afford”
https://www.npr.org/2020/09/17/913093387/why-cant-america-make-enough-n95-masks-6-months-into-pandemic-shortages-persist
Micron Technologies has initiated manufacturing of made in Canada N95 Model 8800 masks at our state-of-the-art manufacturing facility in Delta, BC .. The Company is currently manufacturing and distributing under its Class 1 Medical Devices pursuant to the Company’s Medical Device Establishment License from Health Canada and Medical Device Registration with the US Food and Drug Administration.
The Company’s N95 Model 8800 respirators are currently CSA and FDA approved and undergoing testing and awaiting medical-grade certification from the National Institute for Occupational Safety and Health (NIOSH). N95 masks are the gold standard of personal protective equipment, particularly among healthcare professionals. They provide the highest level of protection for the user and those in close proximity to infected patients. N95 masks are both high in demand and low in supply worldwide. It is the intent of the Company to have its N95 respirators CSA, FDA and NIOSH certified and sold to medical professionals and citizens to help stop the spread of COVID-19.
About Beyond Medical
Beyond Medical is an industrial/technology company with a manufacturing facility located in Delta, British Columbia . The Company is developing its Organivore and Pharmavore waste digesters using its proprietary technology. The Company, through its subsidiary Micron Technologies, is also manufacturing medical grade facemasks compliant with ASTM F2100 Standards. Beyond Medical currently has a non binding LOI to acquire Kayan Health
About Kayan Health
Kayan Health’s proprietary AI-powered health communications platform helps doctors streamline communications with their patients and remotely monitor them. Additionally, Kayan Health’s proprietary platform allows patients to schedule virtual consultations with their physicians and communicate with them through chat, audio and video calls. The platform also integrates with wearable devices and diagnostic tools that delivers both patients and doctors with greater visibility into the patient’s health and provides them with proactive automated alerts such as elevated blood pressure and heart rates. Storing all this data in a holistic patient profile that is integrated with the clinic’s electronic health systems – ensuring their patients’ health records are up to date, accessible by all, helps deliver better care and enables clinics to generate more revenue.
Kayan Health’s platform has already been deployed in multiple clinics in the United States and is projected to reach $5M in revenue by 2022. Kayan Health’s proprietary platform was developed by the founders of HeyDoc!, a telehealth app launched in 2016 that had supported over 3,000 patients globally. Kayan Health is a privately held company based in Toronto, Ontario .
Neither the Canadian Securities Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Canadian Securities Exchange) accepts responsibility for the adequacy or accuracy of this release. The Canadian Securities Exchange has not in any way passed upon the merits of the transactions proposed herein and has neither approved nor disapproved the contents of this press release.
FORWARD LOOKING STATEMENTS:
All information contained in this news release with respect to the Company and Kayan Health was supplied by the parties, respectively, for inclusion herein, and the Company and its respective directors and officers have relied on Kayan Health for any information concerning such party.
This release includes certain statements that may be deemed “forward-looking statements”. All statements in this release, other than statements of historical facts, that address events or developments that the Company expects to occur, are forward-looking statements. Forward-looking statements are statements that are not historical facts and are generally, but not always, identified by the words “expects”, “plans”, “anticipates”, “believes”, “intends”, “estimates”, “projects”, “potential” and similar expressions, or that events or conditions “will”, “would”, “may”, “could” or “should” occur. Although the Company believes the expectations expressed in such forward-looking statements are based on reasonable assumptions, such statements are not guarantees of future performance and actual results may differ materially from those in the forward-looking statements. Factors that could cause the actual results to differ materially from those in forward-looking statements include market prices, continued availability of capital and financing, and general economic, market or business conditions. Investors are cautioned that any such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements.Forward-looking statements are based on the beliefs, estimates and opinions of the Company’s management on the date the statements are made. Except as required by applicable securities laws, the Company undertakes no obligation to update these forward-looking statements in the event that management’s beliefs, estimates or opinions, or other factors, should change.
SOURCE Beyond Medical Technologies Inc.
![]() View original content to download multimedia: http://www.newswire.ca/en/releases/archive/February2021/23/c9523.html
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/February2021/23/c9523.html
News Provided by Canada Newswire via QuoteMedia
The Power Play by The Market Herald has announced the release of new interviews with Reyna Gold, Universal Ibogaine, NewRange Gold, Fabled Copper, and Fandom Sports Media discussing their latest press releases
The Power Play by The Market Herald provides investors with a quick snapshot of what they need to know about the company’s latest press release through exclusive insights and interviews with company executives.
Reyna Gold (REYG) has announced multiple high-grade gold sample results from a surface and trench sampling program at its La Gloria Property in Sonora, Mexico. A total of 1,252 samples results have been received with an initial focus on the Main Zone area where drilling will commence in February 2022. CEO Michael Wood sat down with Caroline Egan to discuss the results, share news of the company’s TSX Venture listing, and highlight what lies ahead for Reyna Gold.
For the full interview with Michael Wood and to learn more about Reyna Gold’s latest results, click here.
Universal Ibogaine (IBO) has appointed Nick Karos as CEO on an interim basis, effective immediately. Mr. Karos is based in Los Angeles, USA, and is a seasoned financier. His career includes senior roles with several US investment banks and most recently as CEO of Private Trading Group, which has provided business development and capital raising services for several successful start-up ventures. Nick Karos sat down with Caroline Egan to discuss the appointment.
For the full interview with Nick Karos and to learn more about Universal Ibogaine’s change in leadership, click here.
NewRange (NRG) has sampled up to 47.34 g/t gold from its Pamlico Project in Nevada. The findings from the mapping and sampling program indicate widespread gold mineralization in the project’s historic Central Mine. A total of 55 of 67 samples returned gold values greater than 0.1 g/t gold, 29 were greater than 1 g/t gold and 13 assayed more than 5 g/t gold. President and CEO Robert Archer spoke with Caroline Egan about the results.
For the full interview with Robert Archer and to learn more about NewRange Gold’s sampling results, click here.
Fabled Copper (FABL) has announced the first results of 2021 surface field work on its Muskwa Copper Project in Northwestern British Columbia. Of the 16 surface samples collected, 5 reported greater than 10 per cent copper and 2 reported greater than 20 per cent copper (1 per cent copper = 22.20 pounds). Peter J Hawley, President and CEO sat down with Caroline Egan to discuss the results.
For the full interview with Peter Hawley and to learn more about Fabled Copper’s latest results, click here.
Fandom Sports (FDM) has launched its peer-to-peer (P2P) sports wagering platform after receiving approval to provide Skrill and Neteller payment services. Wagering customers are now able to fund their accounts to facilitate P2P wagering on Esports and sports. The platform is fully integrated with payment solutions and the company’s banking partners. Fandom Sports CEO and President David Vinokurov sat down with Caroline Egan to discuss the launch.
For the full interview with David Vinokurov and to learn more about Fandom Sports’ P2P sports wagering platform, click here.
Interviews for The Power Play by The Market Herald are released daily. To learn more about the companies featured in The Power Play or to explore our other interviews visit The Power Play by The Market Herald.
About The Market Herald
The Market Herald Canada is the leading source of authoritative breaking stock market news for self-directed investors. Our team of Canadian markets reporters, editors and technologists covers the entire listed company universe in Canada. We cover over 3,985 businesses, their people, their investors, and their customers. We write the stories that move the Canadian capital markets.
DISCLAIMER: Report Card Canada Media Ltd. (“Report Card”) is a wholly-owned subsidiary of Market Herald Limited, an Australian company (“Market Herald”). Report Card is not an advisory service, and does not offer, buy, sell, or provide any other rating, analysis or opinion on the securities we discuss. We are retained and compensated by the companies that we provide information on to assist them with making information available to the public. All information available on themarketherald.ca and/or this press release should be considered as commercial advertisement and not an endorsement, offer or recommendation to buy or sell securities. Report Card is not registered with any financial or securities regulatory authority in any province or territory of Canada, will not be performing any registerable activity as defined by the applicable regulatory bodies and do not provide nor claim to provide investment advice or recommendations to any visitor of this site or readers of any content on or originating from themarketherald.ca. Market Herald and/or its affiliates and/or their respective officers, directors or employees may from time to time acquire, hold or sell securities and/or commodities and/or commodity futures contracts in certain underlying companies mentioned in this site and which may also be clients of Market Herald’s affiliates. In such instances, Market Herald and/or its affiliates and/or their respective officers, directors or employees will use all reasonable efforts to avoid engaging in activities that would lead to conflicts of interest and Market Herald and/or its affiliates will use all reasonable efforts to comply with conflicts of interest disclosures and regulations to minimize any conflict. All the information on this document and/or the website – themarketherald.ca – is published in good faith and for general information purpose only. Report Card does not make any warranties about the completeness, reliability, and accuracy of this information. Any action you take upon the information you find on this document and/or website (themarketherald.ca) is strictly at your own risk. Report Card will not be liable for any losses and/or damages in connection with the use of our website. From our website, you can visit other websites by following hyperlinks to such external sites. While we strive to provide only quality links to useful and ethical websites, we have no control over the content and nature of these sites. These links to other websites do not imply a recommendation for all the content found on these sites. Site owners and content may change without notice and may occur before we have the opportunity to remove a link which may have gone ‘bad’. Please be also aware that when you leave our website, other sites may have different privacy policies and terms which are beyond our control. Please be sure to check the Privacy Policies of these sites as well as their “Terms of Service” before engaging in any business or uploading any information.
CONTACT:
The Market Herald
Brianna Anthony
brianna.anthony@themarketherald.ca
themarketherald.ca
SOURCE: The Market Herald
News Provided by ACCESSWIRE via QuoteMedia
(TheNewswire)
Universal Ibogaine Inc. (TSXV:IBO) (“UI” or the “Company”) announces that Rami Batal has been replaced as Chief Executive Officer (“CEO”) of the Company and its subsidiaries. For the present time, the Company has appointed Nick Karos as CEO, effective immediately. Mr. Karos had recently been appointed as a Capital Markets consultant to the Company
“We are grateful for Rami’s leadership and many contributions,” said Chief Ian Campbell, Chair of the UI Board of Directors. “We wish Rami all the best.”
Mr. Karos is based in Los Angeles, USA, and is a seasoned financier. His career includes senior roles with US investment banks, including serving at Piper Jaffray as head of Nasdaq Trading and Agency Services, and most recently as CEO of Private Trading Group, which has provided business development and capital raising services for several successful start-up ventures.
Mr. Karos said “I’m very excited to work with the talented partners at UI to move towards our goal of conducting clinical trials with Health Canada. Universal Ibogaine‘s goal is to transform the addiction treatment model using ibogaine as the cornerstone of our new addiction treatment protocol. Addiction is a disease that affects society like none other, and we believe our ground-breaking protocol will give patients and families a better path towards long-term recovery.”
The Board of Directors will immediately begin an executive search process for selecting a new CEO. Mr. Karos remains a short list candidate for the long term position.
About Universal Ibogaine Inc.
UI is a life sciences company, with a goal to develop a platform of addiction treatment clinics, which may eventually use ibogaine as a primary modality for the interruption and ideally cessation of addictions to primarily opioids such as oxycodone, heroin, fentanyl, as well as alcohol, cocaine, and other stimulants.
UI separately plans to clinically develop ibogaine, a natural plant substance, as an authorized addiction interruption medicine for the treatment of Opioid Use Disorder. A Clinical Trial Application to Health Canada is being developed to undertake clinical trials in Canada, aimed at proving the safety and efficacy of the use of ibogaine for this purpose. In the longer term, UI plans to introduce ibogaine into the addiction treatment protocols to be used in its’ future facilities.
NEITHER THE TSX VENTURE EXCHANGE NOR ITS REGULATION SERVICES PROVIDER (AS THAT TERM IS DEFINED IN THE POLICIES OF THE TSX VENTURE EXCHANGE) ACCEPTS RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This news release may contain forward-looking statements and information. Forward-looking information is frequently characterized by words such as “plans”, “expect”, “project”, “intend”, “will”, “believe”, “anticipate”, “estimate”, “scheduled”, ”potential”, or other similar words, or statements that certain events or conditions “may”, “should” or ”could” occur. The forward-looking statements and information are based on certain key expectations and assumptions made by UI. Although UI believes that the expectations and assumptions on which the forward-looking statements are based are reasonable, undue reliance should not be placed on the forward-looking statements because UI can give no assurance that they will prove to be correct.
Since forward-looking statements address future events and conditions, by their very nature they involve inherent risks and uncertainties. Actual results could differ materially from those currently anticipated due to a number of factors and risks, which include, but are not limited to, risks that required regulatory approvals are not obtained. The reader is cautioned that assumptions used in the preparation of such information, although considered reasonable by UI at the time of preparation, may prove to be incorrect and readers are cautioned not to place undue reliance on forward-looking information, which speaks only to conditions as of the date hereof. UI does not undertake any obligation to release publicly any revisions to forward-looking information contained herein to reflect events or circumstances that occur after the date hereof or to reflect the occurrence of unanticipated events, except as may be required under applicable securities laws.
For further information:
Investor Relations: Dugan Selkirk – IR Manager
dugan.selkirk@universalibogaine.com
Media Contact: Cathy Fernandes – VP, Marketing & Communications cathy.fernandes@universalibogaine.com
Related Links
https://universalibogaine.com
Copyright (c) 2022 TheNewswire – All rights reserved.
News Provided by TheNewsWire via QuoteMedia
The COVID-19 global pandemic shook traditional healthcare structures on a global scale in 2020. There has been an urgent need for integrated systems to manage the influx of patients and new social distancing measures, which forced many to delay seeking in-person medical attention. In identifying this gap in the healthcare system, digitally driven telemedicine companies are leading the way to innovate and improve healthcare for all in this increasingly digital age.
By 2022, the global healthcare market is forecasted to grow to US$11.9 trillion, with the digital healthcare sector to grow by a projected compound annual growth rate (CAGR) of 38 percent by 2026. In dealing with the aftermath of this pandemic, investing in healthcare and wellness technology is investing in the healthcare systems we’ve needed for a long time.
Cloud DX (TSXV:CDX, OTCQB:CDXFF) is a digital health company focused on providing sophisticated solutions for advanced healthcare providers through its precision vital sign monitoring platform, software and mobile apps. The company’s commitment is to the improved accessibility of healthcare through virtual care and digital medicine across Canada and the United States.
The company combines core competencies in biomedical hardware engineering, cloud-based medical device architecture, algorithm-based result generation, regulatory approval experience and internationally ISO certified quality management. This combination has helped Cloud DX develop optimized medical devices and software for a more value-added patient experience. This level of innovation in telehealth technology strategically positions Cloud DX as a leader in vital sign data monitoring and integration.
Unlike many other telemedicine companies, Cloud DX works to invent new medical devices and get them cleared by regulators. This invention aspect allows the company to create more intuitive and innovative devices at potentially lower costs for its users. “Because we control that entire customer experience, we can optimize it and make it better for everybody. That, in fact, is our mission,” Cloud DX CEO Robert Kaul stated.
Cloud DX has partnered with healthcare providers like the Blessing Health System and virtual care companies like Curatio Inc, co-creator of the Stronger Together app, to provide remote patient monitoring and telehealth solutions to COVID-19’s short term symptoms and its long term complications. This new wave of virtual care technology addresses limitations to existing healthcare structures and helps meets the demand for medical aid in light of strained traditional healthcare systems and broad social distancing measures throughout North America.
In August 2014, Team Cloud DX was confirmed as a top 10 finalist in the Qualcomm Tricorder XPRIZE out of 330 entrants. This three-year global competition offered a US$10 million prize purse to be distributed among the top 3 teams who could develop an autonomous vital sign data collection and remote diagnostic platform inspired by the famous medical “Tricorder” from the TV show Star Trek. Cloud DX’s entry, the VITALITI platform, was successful at diagnosing up to 19 separate health conditions, all by itself. The company, which was the only competitor representing Canada, received the first XPRIZE Bold Epic Innovator Award in April 2017. All the winning XPRIZE Teams share access to a multi-million dollar pool of grant funding provided by the Qualcomm Life Foundation and the Roddenberry Foundation to bring their inventions to market, administered by the University of California San Diego.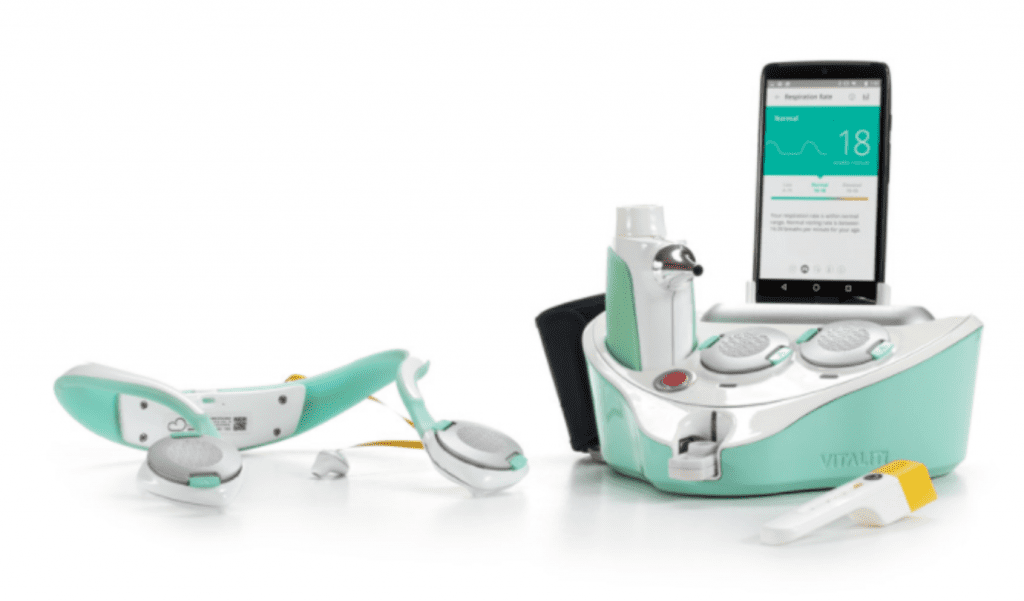
XPRIZE winning VITALITI Tricorder by Cloud DX
Cloud DX is commercializing three inventions based on their XPRIZE winning Tricorder. The Pulsewave 2.0 wrist cuff monitor can uniquely measure accurate blood pressure and average breathing rate simultaneously. The wearable VITALITI continuous vital sign monitor streams all the main vital signs including multi-lead ECG and non-invasive blood pressure to the cloud in real time, while their AcuScreen CA cough analysis smartphone application can detect respiratory disease based on the sound of a person coughing, and is in clinical trials to screen for tuberculosis, expected to be completed by 2022.
Immediate plans for the company include an intended public listing on the Toronto Venture Stock Exchange in early 2021 and the advancement of its innovation pipeline. Its strong patent portfolio strategically positions the company for significant investor potential and technological expansion.
Cloud DX’s management team combines years of experience in medical care, finance, risk management and web-based engineering. This dedicated team primes the company for significant telehealth advancement, product development and revenue growth.
The Cloud DX Connected Health platform combines integrated telehealth monitoring software and high-tech medical hardware. Patients can record their vital signs, complete important surveys to answer questions from a personalized care team, access a curated health feed customized to their condition and have full control over their full patient history through the Cloud DX patient portal.
Cloud DX Connected Health Kit
Additionally, a typical Connected Health Kit comes equipped with a custom Android tablet, a Pulsewave wrist cuff blood pressure monitor, a wireless Bluetooth scale and a wireless pulse oximeter. Smart software in the privacy-compliant Microsoft Azure cloud detects changes to vital signs, survey answers or device use patterns and notifies caregivers so that immediate interventions can occur, improving health outcomes. This virtual care paradigm is an exciting innovation, aiming to answer the demands of a rapidly changing digital medicine landscape.
The Cloud DX Pulsewave health monitor wrist cuff is a unique, connected medical device that allows users to immediately see their heart activity with clinical accuracy. The comfortable wrist cuff transmits the raw pulse wave signal to Cloud DX servers for analysis, producing heart rate, blood pressure, pulse variability, average breathing rate and a proprietary cardiac anomaly score.
“Total Anomaly Score is a novel screening tool that appears to have significant potential for identifying cardiac diseases. Possible clinical uses for this score are under active investigation at Cloud DX,” Chief Medical Officer Dr. Sonny Kohli noted.
The company expects to launch Pulsewave 2.0, a major upgrade to their original wrist cuff, in 2021. The new device is wireless, battery powered, with an on-board accelerometer and gyroscope, improving performance. Production of Pulsewave 2.0 is supported by a non-dilutive $1.75 million co-investment from the NextGen Manufacturing Supercluster.
Robert Kaul has over 30 years of experience as an entrepreneur, and Cloud DX is his 6th startup. With a BSc in Biology and a certificate in Operations Strategy from MIT-Sloan, Kaul is recognized as an expert in creating and inspiring highly capable teams.
Dr. Sonny Kohli is an Attending Physician in Intensive Care and Internal Medicine at Oakville Trafalgar Memorial Hospital and an assistant clinical professor (Adjunct) of Medicine at McMaster University. In 2008/09, Dr. Kohli was an Astronaut Candidate at the Canadian Space Agency (CSA), where he was awarded a Scholarship to the Johnson Space Center.
Anthony Kaul is the founder & CEO of Higher Bracket Online Media, Canada’s only online resource for $100K careers. He has over 25 years of corporate leadership experience and is responsible for all day-to-day operations at Cloud DX including HR, Purchasing, Finance, Sales, Marketing, and much more.
With more than 20 years of finance, accounting and strategic planning experience, Simon has worked in professional services, industrials, mining and resources, banking, and e-commerce sectors in Canada and internationally. In his new role, Simon will lead Cloud DX‘s finance department including finance, tax, capital, investor relations (IR) and strategic growth initiatives. Former CFO Stephanie Bird will continue to be available as an Executive Consultant. Simon joins Robert Kaul, CEO and Anthony Kaul, COO as a member of the Executive Team.
Nick Mulder has over 18 years of experience building web-based systems and over 12 years of experience building high-performance teams. Before joining Cloud DX, Mulder was the senior engineering lead that founded and grew the product and engineering team at Shopify Plus in Waterloo, growing the team from 4 to 400 in three years.
Sara Ross-Howe brings 16 years of experience developing advanced technology solutions for consumers. She started her career managing a team of scientists applying machine learning techniques to biomedical diagnostics. More recently, she spent over a decade managing a technology group creating audio and video recording solutions and cloud transcription for national court systems.
BioHarvest Sciences (CSE:BHSC), a biotechnology innovator, is targeting the international market demand for natural products that provide consumers with functional health and wellness. This includes consumer products ranging from foods enriched with active ingredients like antioxidants to cleaner and more consistent cannabis products. BioHarvest Sciences has developed biofarming, a proprietary breakthrough patented technology, capable of naturally-producing the active ingredients of a plant without having to grow the plant itself. The company has already proven the technology in the rapidly growing nutraceuticals market focusing on dietary supplements and the functional food and beverage ingredients market. Products such as BioHavest Sciences’ VINIA®, which is based on red grapes, has clinically- proven functional benefits, has already positively impacted the lives of thousands of Israelis and is approved for sale in the US.
The global cannabis market is expected to reach US$66.3 billion by the end of 2025, according to a report by Grand View Research. A large portion of the growth has been driven by the adoption of cannabis in the pharmaceutical industry as new products have been developed to treat severe medical conditions such as cancer, Parkinsons, Alzheimers and arthritis. However, issues with consistent cannabis supply can affect the long-term growth of the industry as more countries initiate cannabis programs and legislative reforms, posing potential difficulties for licensed producers to provide enough high-quality products to meet the demand.
BioHarvest Sciences Inc. believes that its biofarming technology is the solution to the cannabis supply and consistency problem. The technology isolates the active ingredient cells from the cannabis plant before multiplying (growing) them in the biofarming process. The technology can do this without using any solvent extraction, genetic modification or synthetic molecular processing techniques. To facilitate its biofarming operation, BioHarvest Sciences Inc. has built a production facility that can produce approximately one ton of active cannabis ingredient powder (equivalent to the cannabis plant dried bud) per year in a 100-square-meter space. The company intends to increase its production to 10 tons per year by 2022.
As of September 2019, the company produced its first cannabis cells in suspension with a cannabinoid profile that was identical to the original cannabis plant without growing the plant. Following a B2B business model, BioHarvest Sciences Inc. intends to sell its active ingredients as a powder for repackaging and formulation to its clients. In addition, the company may License the technology in order to accelerate the industry adoption curve.
BioHarvest Sciences has already demonstrated the feasibility and viability of the biofarming technology through its commercially available product called VINIA®. VINIA® is based on many studies which have demonstrated that moderate consumption of red wine every day is able to have a positive impact on one’s overall heart health as a result of wine’s rich polyphenol content, specifically resveratrol. One 400mg capsule of VINIA® contains the same amount of resveratrol contained in one full bottle of Red Wine without the sugar, calories or alcohol found in red wine. Consumers can currently purchase the powder through the VINIA website in a 400-milligram daily dose.
Dr. Rakib is a serial entrepreneur and seasoned executive. He brings extensive experience in multiple industries. Prior to BioHarvest Sciences, Dr. Rakib co-founded Terayon Communication Systems, led the company from inception as its CEO, and managed its growth from $2M to $380M in revenue. Terayon reached a $7B market capitalization in 2000 and was later on acquired by Motorola. Prior to that, Mr. Rakib was a director of engineering at Cadence design systems which acquired Helios S/W where he served as CTO. Dr. Rakib holds a Ph.D. in Mechanical Engineering and a Ph.D. in Applied Mathematics.
Ilan, brings extensive experience in General Management, International Sales & Marketing, Manufacturing & Operations and leadership expertise in building large-scale businesses and billion- dollar brands. For the past 6 years, Ilan served as COO and transitioned to Chief Commercial Officer of Weissbeerger where he played a major leadership role in building a disruptive BIG Data, IOT & Software Company servicing major Beverage players which was recently purchased by ABInBev. Previously, Ilan served an 18-year stint as an International Employee of The Coca-Cola Company, where he played a pivotal role in key senior leadership positions generating significant revenue and profit growth and improving brand health trends across diverse global markets including the United States, China, South East and West Asia and South Africa.
With a Ph.D in Biotechnology and 20+ years of relevant experience, leading substantial research and development programs in both pharma and biotech, Dr. Hagay has lead the development and implementation of BioHarvest’s technology platform since inception. She previously worked in various leadership positions at BTG corporation which was acquired by FERRING Pharmaceuticals. Dr. Hagay specializes in genetic engineering, molecular biology, tissue culture, monoclonal antibodies and clinical trials. She is the author and co-author of several peer-reviewed – published in scientific papers.
David Ryan has extensive experience in investment and public markets. For the past 20+ years, he has been part of in bringing multiple initial public offerings to market. He has helped raise both equity and debt financings for numerous public companies in both primary and secondary financings as well as served on the board of public companies and in various roles.
Mr. Popper was the co-founder and President of MedReleaf Corp., which was acquired in 2018 for $2.5 billion USD. Prior to its acquisition, MedReleaf was one of the largest and most reputable vertically integrated medical Cannabis producers in the world. Mr. Popper brings over 15 years of international partnerships, entrepreneurial ventures, disruptive industry, large-scale project development, engineering and investment experience. He holds a B.Sc. in Civil Engineering, a M.Sc. in Environmental Fluid Mechanics from Stanford University, and an MBA from the Recanati School of Business.
Dr. Azachi brings 20 years of experience in biochemistry, genetic engineering, tissue culture, molecular biology, and clinical & pre-clinical trials. Prior to BioHarvest, He served as technology Director at HealOr Ltd, a Biopharmaceutical company developing topical therapeutics. Prior, he led product development at the research and development department of Colbar LifeScience, a Johnson & Johnson Company. Dr. Azachi holds a Ph.D in microbiology from the Hebrew University of Jerusalem and a Post-Doc in Molecular Biology of the cell from Weizmann Institute of Science.
Michal Sapir brings 30+ years of experience in the medical device, pharma and biotechnology industries. She has previously served as Senior Director of Project Management at ColBar LifeScience Ltd., a Johnson & Johnson Company. She actively participated in FDA meetings in order to define regulatory pathways, FDA inspections and ISO Audits. She has broad experience in clinical and animal studies; and had formerly served as Affiliate Quality Coordinator & Senior Clinical Research Administrator in Eli Lilly (1995-2000). Michal Holds a Master of Science in Biochemistry.
Brady brings to the advisory board 30 years of experience as an integrative and nutritional medicine practitioner and over 25 years in health sciences academia. He is a licensed naturopathic medical physician in Connecticut and Vermont, is board certified in functional medicine and clinical nutrition, and is a fellow of the American College of Nutrition. Dr. Brady has been the Chief Medical Officer of Designs for Health, Inc. and also currently serves as the Chief Medical Officer for Diagnostic Solutions Labs, LLC. He was the long-time Vice President for health sciences and Director of the Human Nutrition Institute and continues to serve as an associate professor of clinical sciences, at the University of Bridgeport in Connecticut. He has published multiple peer-reviewed scientific papers and textbooks related to chronic pain, autoimmunity, and functional gastroenterology. Furthermore. Dr. Brady appeared on the plenary speaking panels of some of the largest and most prestigious conferences in the field including; IFM, ACAM, A4M, ACN, IHS, AANP, AIHM, and many more.
Mr. Tsur is the co-founder of Kamada Ltd, a public company listed on both the NASDAQ and Tel-Aviv Stock Exchange. He served as its Chief Executive Officer and on its Board of directors since the Company’s inception in 1990. He currently serves as Deputy Chairman of the Board.
He also serves as the Chairman of Kanabo Group Plc, a company listed on the London Stock Exchange, which focuses on distributing Cannabis-derived products for medical patients and non-THC products for CBD consumers.
Steven currently sits on the Board of Directors of two life science companies, one not for profit, and is a member of the University of Maryland’s Bioengineering Department Advisory Board. He provides strategy and implementation advice to several organizations on various topics from commercial efforts through operations, business development, product development, portfolio planning to the establishment of international operations. Previously Steve was Head of Biologicals at Cipla Ltd., CEO of Cipla BioTec, President of Glycominds Ltd, EVP of Adamas Pharmaceuticals Inc., CEO of GeneOs Ltd, CEO at DNA Sciences, and was a division President of Monsanto. Steven also worked with McKinsey & Co., and Proctor & Gamble Corporation.
Hadfield brings 40 years of scientific experience to the Canadian-Israeli biotech firm, which has developed and patented a plant bio-cell technology, called BioFarming, capable of growing the active and beneficial plant based ingredients at industrial scale, without the need to grow the plant itself.
INN does not provide investment advice and the information on this profile should not be considered a recommendation to buy or sell any security. INN does not endorse or recommend the business, products, services or securities of any company profiled.
The information contained here is for information purposes only and is not to be construed as an offer or solicitation for the sale or purchase of securities. Readers should conduct their own research for all information publicly available concerning the company. Prior to making any investment decision, it is recommended that readers consult directly with BioHarvest Sciences Inc. and seek advice from a qualified investment advisor.
(TheNewswire)
Calgary, AB – TheNewswire December 2, 2021 Universal Ibogaine Inc. (TSXV:IBO) (” UI ” or the ” Company “), a life sciences company with a mission to research and deliver medicalized ibogaine-centered addiction care, is pleased to announce an expansion of its’ Executive team, and welcomes Nick Karos as its new Director Capital Markets.
Mr. Karos is based in Los Angeles, USA, and is a seasoned financier. His career includes senior roles with US investment banks, including serving at Piper Jaffray as head of Nasdaq Trading and Agency Services, and most recently as CEO of Private Trading Group, which has provided business development and capital raising services for several successful start-up ventures.
Mr. Karos’ mandate will include expanding awareness of UI in the US-based investment community (including institutional investors, family offices, high net worth individuals and investment advisors), securing sources of expansion capital, as well as identifying business development opportunities in the addiction treatment industry. UI is currently in the process of finalizing an application to be listed for trading in the USA on the OTC QB market.
UI also advises that it has granted incentive stock options (” Options “) to members of its recently expanded Executive team, as well as to several key, long-serving consultants to UI, and two new members of its’ Board of Directors who joined UI effective October 18, 2021, as follows:
Chief Clinics Officer (Ian Rabb, joined UI November 15)
3,000,000
Chief Financial Officer (Greg Leavens)
2,000,000
Director – Capital Markets (Nick Karos)
2,000,000
Board members (David Danziger and Anthony DeCristofaro, joined UI October 18)
400,000
Consultants
1,450,000
8,850,000
The grants are made under the Company’s 20% Fixed Stock Option Plan, which based on the total shares outstanding as at the date of the Company’s Amalgamation on August 31, 2021, has a cap of 38.1 million shares issuable for the exercise of Options and Performance Shares.
None of the current grantees have previously been issued any UI Options, and each Option will entitle the holder to acquire one UI common share at an exercise price of $0.25 per share (which was the issue price of the August 31, 2021 go-public Offering). Entitlement to exercise the Options will vest over a term of 3 years (1/3 per year) for 4,850,000 of the Options and 2,000,000 Options will vest over a term of 2 years. For Mr. Karos, the vesting period is one year, and 1,150,000 of his 2,000,000 Option grant (58% of his total) will only vest upon achieving performance-based milestones. The term to expiry of the Options will be 5 years, except for 3,000,000 Options which will have a term of 10 years.
Dr. Rami Batal, UI’s CEO noted “As a life sciences company, we are an integral participant in the knowledge economy. While we have aimed to minimize cash compensation costs, the importance for UI of recruiting and retaining top talent is paramount, particularly in today’s competitive environment. Our team, including members of our Board of Directors who continue to provide both governance and operational guidance, are relentless in their quest to make UI successful. The recent addition of Anthony DeCristofaro and David Danziger enhanced our Board of Directors and strengthened our business and financial acumen. Dr. Ian Rabb, who joined us in mid-November, is making considerable strides in shaping our innovative addiction care model, which is to be piloted at the Kelburn Clinic in Winnipeg. Mr. Nick Karos, our exciting new addition to the UI team, will augment our corporate finance and capital markets expertise and experience, and bolster our access and effectiveness in the ever-critical US market environment.”
About Universal Ibogaine Inc.
UI is a life sciences company, with a goal to develop a platform of addiction treatment clinics, which may eventually use ibogaine as a primary modality for the interruption and ideally cessation of addictions to primarily opioids such as oxycodone, heroin, fentanyl , as well as alcohol, cocaine, and other stimulants.
UI separately plans to clinically develop ibogaine, a natural plant substance, as an authorized addiction interruption medicine for the treatment of Opioid Use Disorder. A Clinical Trial Application to Health Canada is being developed to undertake clinical trials in Canada, aimed at proving the safety and efficacy of the use of ibogaine for this purpose. In the longer term, UI plans to introduce ibogaine into the addiction treatment protocols to be used in its’ future facilities.
NEITHER THE TSX VENTURE EXCHANGE NOR ITS REGULATION SERVICES PROVIDER (AS THAT TERM IS DEFINED IN THE POLICIES OF THE TSX VENTURE EXCHANGE) ACCEPTS RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This news release may contain forward-looking statements and information. Forward-looking information is frequently characterized by words such as “plans”, “expect”, “project”, “intend”, “will”, “believe”, “anticipate”, “estimate”, “scheduled”, ”potential”, or other similar words, or statements that certain events or conditions “may”, “should” or ”could” occur. The forward-looking statements and information are based on certain key expectations and assumptions made by UI. Although UI believes that the expectations and assumptions on which the forward-looking statements are based are reasonable, undue reliance should not be placed on the forward-looking statements because UI can give no assurance that they will prove to be correct.
Since forward-looking statements address future events and conditions, by their very nature they involve inherent risks and uncertainties. Actual results could differ materially from those currently anticipated due to a number of factors and risks, which include, but are not limited to, risks that required regulatory approvals are not obtained. The reader is cautioned that assumptions used in the preparation of such information, although considered reasonable by UI at the time of preparation, may prove to be incorrect and readers are cautioned not to place undue reliance on forward-looking information, which speaks only to conditions as of the date hereof. UI does not undertake any obligation to release publicly any revisions to forward-looking information contained herein to reflect events or circumstances that occur after the date hereof or to reflect the occurrence of unanticipated events, except as may be required under applicable securities laws.
For further information:
Investor Relations: Dugan Selkirk – IR Manager
dugan.selkirk@universalibogaine.com
Media Contact: Cathy Fernandes -VP, Marketing & Communications cathy.fernandes@universalibogaine.com
Related Links
https://universalibogaine.com
Copyright (c) 2021 TheNewswire – All rights reserved.
News Provided by TheNewsWire via QuoteMedia
Sixth Wave Innovations Inc. (CSE: SIXW) (OTCQB: SIXWF) (FSE: AHUH) (“Sixth Wave”, “SIXW” or the “Company”) is pleased to provide an update on commercial deployment of the Affinity™ System (the “System”) for purification of cannabinoids. Green Envy Extracts (customer) will ramp up its production line in Michigan on or about December 15, 2021. Once production commences, SIXW will use the crude extract to complete performance testing on the Affinity System. Testing will occur three weeks earlier than initially planned. SIXW has also completed the manufacture of Affinity™ nano-tech beads for use in the full-size production Affinity™ System scheduled for installation in Michigan. By year-end, SIXW will manufacture three additional bead orders that are slated to complete four production Affinity™ Systems.
“We are very pleased to continue our collaboration with Sixth Wave on the launch of the Affinity™ system,” states Amato Spagnoletti, [Director] of Green Envy. “As a growing multi-state cannabis operator, we believe that our respective technological approaches will yield significant technical and commercial benefits as we commission the first Affinity system with Sixth Wave in Michigan. Per our signed MOU, we plan to purchase a minimum of 3 Affinity™ units for placement at additional locations in the coming months,” he added.
Based on current operating procedures (SOP), the system will be capable of processing approximately 6kg of final high purity distillate every 10hrs of operation. The larger production unit will be capable of producing finished distillate of approximately 14kg/10hrs of operation or more than 30kg/day which surpasses the Company’s 20kg/day goal. Sixth Wave will begin revenue generation with the initial system as soon as optimization trials are completed. A single production system will be capable of generating approximately CAD$2.25M annually based on a 300-day production schedule while netting the licensed producer cost savings/increased yield that should nearly double the Affinity™ related license costs.
Sixth Wave expects to see continued growth in the cannabis sector as State-by-State and potential Federal legalization efforts advance across the United States. The US House of Representatives House Judiciary Committee just passed The Marijuana Opportunity, Reinvestment, and Expungement or the MORE Act. Legalization will drive demand for cannabis processing capacity and support demand for the Affinity™ System.
The Company is in discussions with other early adopter candidates that have a significant presence within the industry in Canada and the United States. Further, SIXW is in joint venture negotiations with an organic cellular growth company that has chosen SIXW to lead its global cannabis product development as well as other high-value plants and roots used in food products, nutraceuticals, and medicines.
About Sixth Wave
Sixth Wave is a nanotechnology company with patented technologies that focus on extraction and detection of target substances at the molecular level using highly specialized Molecularly Imprinted Polymers (MIPs). The Company is in the process of a commercial rollout of its Affinity™ cannabinoid purification system, as well as IXOS®, a line of extraction polymers for the gold mining industry. The Company is also in the development stages of a rapid diagnostic test for viruses under the Accelerated MIPs (AMIPS™) label.
Sixth Wave can design, develop and commercialize MIP solutions across a broad spectrum of industries. The company is focused on nanotechnology architectures that are highly relevant for the detection and separation of viruses, biogenic amines, and other pathogens, for which the Company has products at various stages of development.
For more information about Sixth Wave, please visit our website at: www.sixthwave.com.
ON BEHALF OF THE BOARD OF DIRECTORS
“Jonathan Gluckman”
Jonathan Gluckman, Ph.D., President & CEO
For information, please contact the Company:
Phone: (801) 582-0559
E-mail: info@sixthwave.com
Cautionary Notes
This press release includes certain statements that may be deemed “forward-looking statements” including possible statements regarding the planned use of proceeds and performance of the IXOS®, Affinity™, and AMIPs™ technologies. All statements in this release, other than statements of historical facts, that address future events or developments that the Company expects, are forward-looking statements. Although the Company believes the expectations expressed in such forward-looking statements are based on reasonable assumptions, such statements are not guarantees of future performance, and actual events or developments may differ materially from those in forward-looking statements. Such forward-looking statements necessarily involve known and unknown risks and uncertainties, which may cause the Company’s actual performance and financial results in future periods to differ materially from any projections of future performance or results expressed or implied by such forward-looking statements. In particular, successful development and commercialization of the IXOS®, Affinity™, or AMIPs™ technologies are subject to the risk that they may not prove to be successful, the uncertainty of medical product development, the uncertainty of timing or availability of required regulatory approvals, lack of track record of developing products for certain applications and the need for additional capital to carry out product development activities. The value of any products ultimately developed could be negatively impacted if patents are not granted. The Company has not yet applied for regulatory approval for the use of this product from any regulatory agency.
To view the source version of this press release, please visit https://www.newsfilecorp.com/release/104142
News Provided by Newsfile via QuoteMedia
Investing News Network websites or approved third-party tools use cookies. Please refer to the cookie policy for collected data, privacy and GDPR compliance. By continuing to browse the site, you agree to our use of cookies.



